By Onno Faber
Rare Disease Day is recognized every year on the on the last day of February. It’s a time for the entire rare disease community to come together to raise awareness with the general public and decision-makers about rare diseases and their impact on patients’ lives. Neurofibromatosis affects 1 in 3,000 people overall; NF2 is even more rare (1 in 25,000) and schwannomatosis even more rare (1 in 40,000).
Rare disease research contributes to the development of diagnostic tools, treatments and cures, as well as improved health and social care for patients and their families. This is why the global theme for Rare Disease Day 2018 is research.
We asked NF2 patient Onno Faber to tell us about his journey to a diagnosis and how he leveraged his experience as a Silicon Valley technology entrepreneur to accelerate rare disease research & drug development.
Let’s deal with NF
Today is Rare Disease Day and I feel proud to be part of the NF community. It’s been over three years since I was first diagnosed with NF2. I’m always telling people how grateful I feel to have made it my full-time job and work on helping patients get the most out of their care while creating resources to accelerate the availability of treatments. To me, to “deal with NF” means we have to find a way to live with it – and at the same time work on long-term solutions to eliminate the negative impact on our lives. This is not a matter of any individual tasked to ‘fix things,’ it requires strong collaboration and alignment across the board. This is what I want to help facilitate with our newborn startup called RDMD.
Some of you may already have heard my story. I was a tech startup founder in Silicon Valley, working on a social media company called Taptalk before I was diagnosed with NF2. My issues started with hearing loss on my left side and it took about a year before the final diagnosis was made. After one of my tumors was surgically removed I was able to genetically sequence the tumor tissue with the help of my friends who are pioneering computational genome analysis. Last year, we ended up organizing an NF2+AI+Genomics event (a “Hackathon”), where 300 engineers and scientists came together here in San Francisco to spend their weekend “hacking” on the genetics of NF2. It was one of the craziest things I’ve experienced so far – witnessing over 20 teams present their findings on my genomic data. (Click here to watch a mini-documentary of the NF2 Hackathon event that took place in San Francisco in 2017)
 Fast forward six months, hundreds of phone calls, meetings, and coffee mugs later, a new startup was born to address some of the critical problems rare disease patients and researchers experience. Our mission is to significantly accelerate treatments for patients with rare disease – and we’re starting with NF2. The product we’re developing is designed to help patients improve their care while collectively building the most comprehensive resource for drug development. We are tapping into the expertise of the Children’s Tumor Foundation and their experience with the NF Registry in order to develop it.
Fast forward six months, hundreds of phone calls, meetings, and coffee mugs later, a new startup was born to address some of the critical problems rare disease patients and researchers experience. Our mission is to significantly accelerate treatments for patients with rare disease – and we’re starting with NF2. The product we’re developing is designed to help patients improve their care while collectively building the most comprehensive resource for drug development. We are tapping into the expertise of the Children’s Tumor Foundation and their experience with the NF Registry in order to develop it.
What we learned so far
Rare disease R&D (research & development) is really hard. There are fewer patients, fewer doctors, and fewer researchers, so getting everything in place to run a drug program is extremely expensive and time-consuming. Most people think about treatment development as a process of scientific discovery or new technologies such as gene editing. While this is very important, the largest costs are incurred before and after a compound is actually discovered. Pharmaceutical companies need to spend billions of dollars each year collecting detailed clinical data. Before they can get a drug approved, they need to know things like the symptoms, diagnostic journey, genetics, medical records, and treatment history. In order to accelerate and increase the probability of successful treatments, we need to turn the model on its head and start building these resources right now. The best part is we’re building it in a way that empowers you to contribute.
What we’re doing
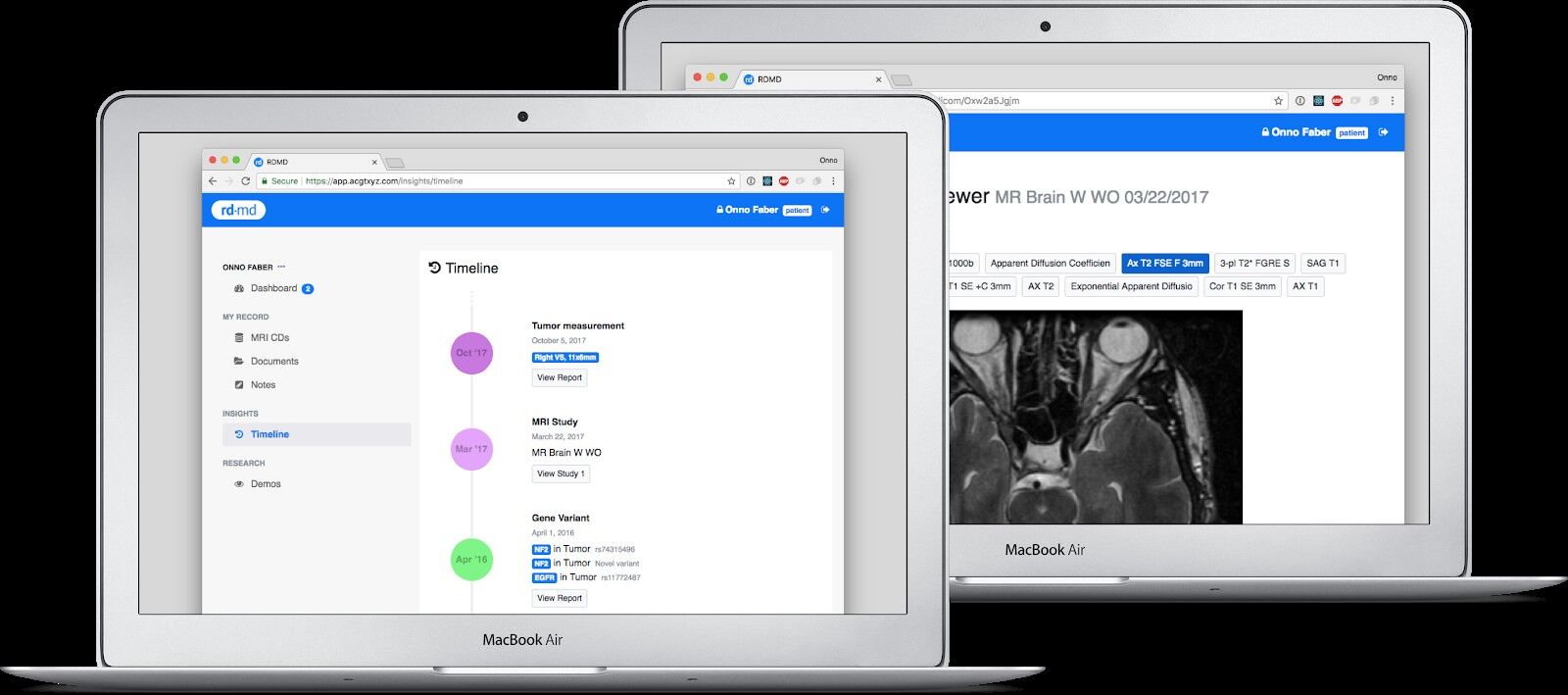
RDMD is a clinical R&D platform for rare disease. We have two goals: 1) to help patients manage their own care, while 2) setting up missing research infrastructure for better, faster, and cheaper drug R&D in the longer term.
Currently, patients, families, and clinicians spend a lot of time and energy on the phone, faxing, mailing, copying PDFs, CDs, reports, and files. We are building technology to streamline that process, so patients can send their complete profile to new doctors, view MRI scans online, and easily set up remote consultations with an expert in their condition. All the data is curated to not just make better decisions on care, but also generate real-world data and evidence that a researcher, a biopharma company, or the FDA could use in drug development. We work with specialists, advisors, and foundations to design these products to make them highly relevant to specific conditions, like NF2 or Schwannomatosis, because what’s needed for an R&D program in each of these conditions may be very different.
After the Genomics “Hackathon” we realized getting more patients involved will help accelerate research even further and our plan to offer patients research-grade genetic sequencing is still in our plans. At the same time, to get the maximum information out of the genomic data, we also need clinical data to be used as the background reference for symptoms. With genetic sequencing costs declining rapidly, it is becoming more realistic to combine high quality clinical data and genomic data for drug discovery efforts.
A lot of our work focuses on making sure that the data and insights generated are adequately robust, relevant, and compliant to be truly useful to R&D. A lot of security, compliance, and privacy protocols are needed to ensure that the data collected is accurate, secure, and properly managed.
What happens next
The NF community and CTF has been very involved and supportive throughout this journey and they are the real reason we got this far. In the coming months we will be working hard to get our project off the ground and with the help of the community, and all of you I believe we can make a difference. We’re just getting started and I could not be more excited to be on this journey with you, thank you. Visit RDMD to learn more – let’s deal with NF together!
Onno Faber will be speaking at the Children’s Tumor Foundation NF Forum in Atlanta, GA on May 5, 2018.

