The April 2020 approval of Koselugo (selumetinib) as the first FDA-approved treatment for NF1 sparked more interest and attention toward NF. As our understanding of NF has grown, more potential treatments are now within our grasp, and effective preclinical testing will better predict success once the drug moves into clinical trials.
The Children’s Tumor Foundation pledges to accelerate this path to drug discovery by constructing an NF-focused Preclinical Hub to supercharge the development of NF treatments.
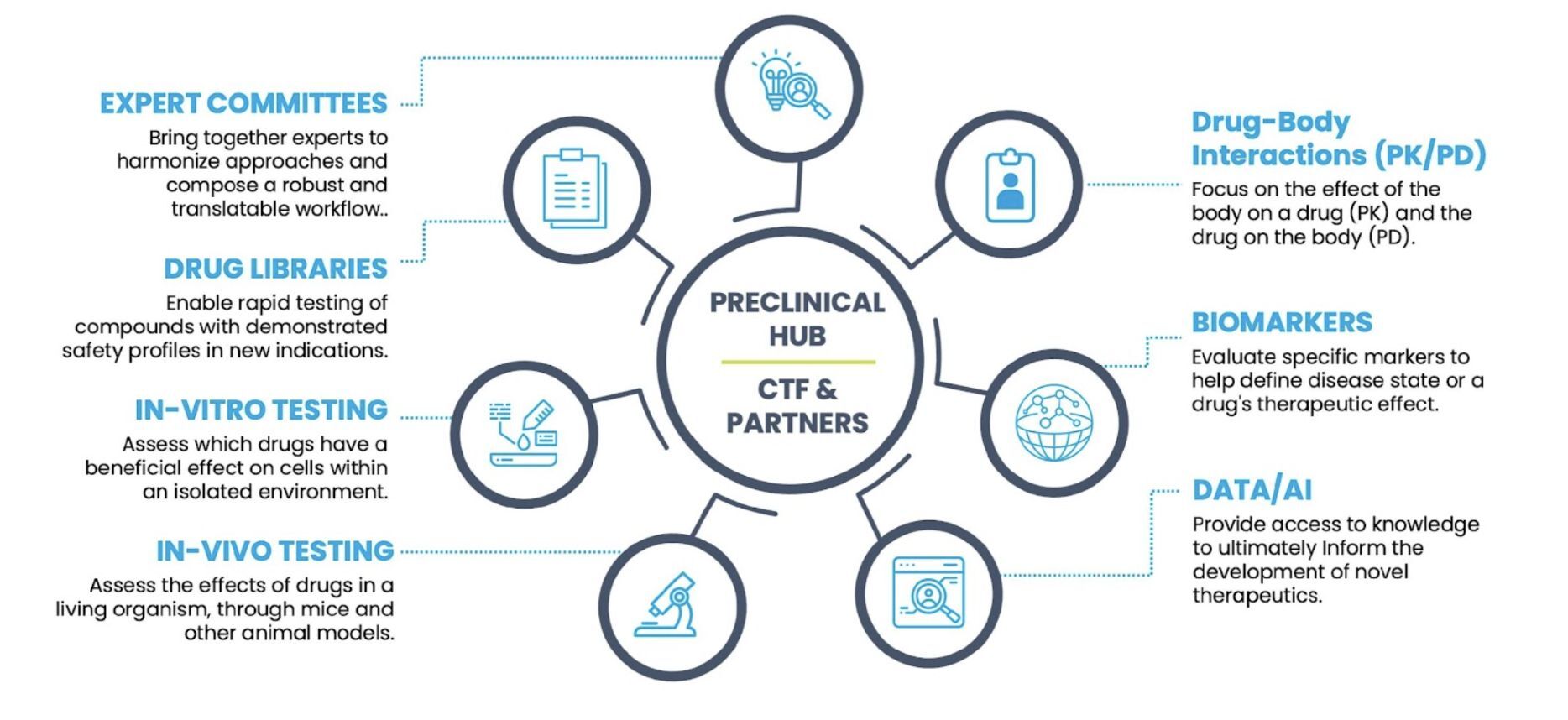
CTF’s Preclinical Hub is a commitment to speed innovation and build bridges across academia, industry, and the medical community. The Preclinical Hub will streamline access to the best preclinical models and guide each potential treatment through a swift and appropriate screening journey. This solution will pave the way for the efficient selection of NF-relevant treatments for clinical trials.
The CTF Preclinical Hub is a life-changing innovation that uses CTF’s network of experts, collaborators, and resources to close major gaps.
The Preclinical Hub will offer:
- Negotiated Master Service Agreements
- Predetermined protocols and tests
- Access to CTF models, data tools, libraries, and biomarkers
With a proposed investment of $7 million over five years, our Preclinical Hub will catalyze drug discovery, increase testing, and speed the approval of potential treatments. This isn’t just a resource—it’s a revolution in NF treatment and research.
CALL FOR MODELS
As part of the implementation of its strategic plan, CTF is in the process of building the Preclinical Hub, a service-for-fee model where high-quality NF (including NF1, and all types of SWN, including NF2-SWN) in vitro and in vivo assays can be performed upon request by anyone at a dedicated CRO.
To enter the Hub, your model must be already characterized and validated. If you and your institution are willing to contribute cell lines and/or animal models to the Hub, please email Irene Morganstern (imorganstern@ctf.org), Director of Preclinical Initiatives, before Nov 1st, 2023.
Please contain the following:
- Description of the model and relative references (origins of the model, published)
- Characterization and validation data (preferably with known reference compounds)
- Availability and current status of the model/timelines for sharing the model
- Confirmation that your TTO is willing to grant a non-exclusive commercial license to the model

